Alzheimer’s disease is a complex condition that affects memory and thinking abilities. While scientists are still uncovering its exact causes, inflammation and oxidative stress are believed to be key factors. Imagine inflammation as a kind of internal swelling in the brain, and oxidative stress as damage caused by harmful molecules. These processes can harm brain cells and lead to the formation of plaques and tangles, which are hallmarks of Alzheimer’s. Unhealthy lifestyle habits like poor diet, lack of exercise, and chronic stress can worsen these problems. Additionally, exposure to certain toxins in our environment, like heavy metals and chemicals, may also play a role. Plus, deficiencies in essential vitamins and hormones might contribute to the risk of developing Alzheimer’s. By understanding these factors, we can take steps to support brain health and reduce our risk of Alzheimer’s disease.
Alzheimer’s disease is a multifactorial neurodegenerative condition characterized by progressive cognitive decline and memory loss. While its exact cause remains elusive, research suggests that inflammation and oxidative stress play significant roles in its development and progression. Inflammation in the brain, often referred to as neuroinflammation, leads to the activation of immune cells and the release of pro-inflammatory molecules, contributing to neuronal damage and dysfunction. Oxidative stress occurs when there is an imbalance between the production of free radicals and the body’s antioxidant defenses, resulting in cellular damage. These processes can disrupt neuronal communication and contribute to the formation of characteristic Alzheimer’s plaques and tangles in the brain. Additionally, various lifestyle factors, including poor diet, lack of exercise, chronic stress, and inadequate sleep, can exacerbate inflammation and oxidative stress, further increasing the risk of Alzheimer’s disease. Moreover, environmental factors such as exposure to heavy metals and chemicals may also contribute to neurodegeneration. Furthermore, deficiencies in essential vitamins and micronutrients, hormonal imbalances, and genetic predispositions are believed to play roles in the development of Alzheimer’s disease. Understanding these multifaceted aspects of the disease is crucial for developing effective prevention and treatment strategies.
Oxidative stress is a key mechanism in the pathogenesis of Alzheimer’s disease (AD).
Oxidative stress, characterized by an imbalance between antioxidants and oxidants, is a major contributor to the neurodegeneration observed in AD. Increased production of reactive oxygen species (ROS) and free radicals can damage lipids, proteins, and DNA in the brain, leading to cellular dysfunction and death.[1][2][3]
Oxidative stress is an early and prominent feature of AD, occurring even before the accumulation of amyloid-β (Aβ) and neurofibrillary tangles, the hallmark pathological hallmarks of the disease.[4] Studies have found increased markers of oxidative damage in the brains, cerebrospinal fluid, plasma, and peripheral tissues of individuals with mild cognitive impairment and early-stage AD.[4][5]
Genetic and lifestyle risk factors for AD, such as mutations in presenilin genes and the apolipoprotein E genotype, are associated with increased oxidative stress.[2][4] Conversely, interventions that reduce oxidative stress, like caloric restriction, exercise, and antioxidant supplementation, have been shown to promote neuronal survival and potentially reduce the risk or progression of AD.[4][5]
In conclusion, overwhelming evidence from the search results indicates that oxidative stress is a key mechanism underlying the pathogenesis of Alzheimer’s disease, playing a central role in the neurodegeneration observed in this disorder.[1][2][3][4][5]
Citations:
[1] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4840676/
[2] https://jamanetwork.com/journals/jamaneurology/fullarticle/775665
[3] https://www.mdpi.com/1420-3049/24/8/1583
[4] https://academic.oup.com/jnen/article/65/7/631/2646707
[5] https://www.mdpi.com/2076-3921/10/9/1479
Effective antioxidants for brain protection
Based on the search results, several effective antioxidants have been shown to provide neuroprotection and protect the brain:
Antioxidants like carotenoids, vitamin E, ascorbic acid (vitamin C), and flavonoids such as hesperidin can effectively inhibit oxidative stress and lipid peroxidation, thereby preventing brain aging and neurodegenerative diseases like Alzheimer’s disease.[1]
Enzymatic antioxidants in the brain like superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) play a key role in protecting brain cells from oxidative damage.[2]
Non-enzymatic antioxidants such as reduced glutathione (GSH) and thioredoxin also help regulate oxidative stress and maintain brain homeostasis.[2]
Antioxidants that can readily cross the blood-brain barrier, like the pyrrolopyrimidine class of compounds, coenzyme Q10, and vitamin E derivatives, are particularly good therapeutic candidates for neurological disorders.[3]
Combining different types of antioxidants, such as vitamin E and vitamin C, or using antioxidants alongside other neuroprotective agents like iron chelators, may provide synergistic benefits in protecting the brain.[3]
Overall, the search results indicate that a variety of plant-derived and endogenous antioxidants can effectively protect the brain from oxidative stress-induced damage and neurodegeneration.[1][2][4]
Citations:
[1] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7372124/
[2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7582347/
[3] https://jamanetwork.com/journals/jamaneurology/fullarticle/777432
[4] https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2021.729757/full
[5] https://molecular-cancer.biomedcentral.com/articles/10.1186/s12943-022-01668-9
Vitamin C for brain protection
Vitamin C plays an important protective role in the brain and is highly concentrated in certain brain regions like the cerebral cortex, hippocampus, and amygdala.[1][2] Studies have shown several key mechanisms by which vitamin C benefits brain function:
– Vitamin C is a potent antioxidant that scavenges reactive oxygen species and protects the brain from oxidative damage.[1][2][3]
– It is a cofactor for enzymes involved in the synthesis of neurotransmitters like catecholamines and serotonin, supporting proper brain signaling.[1]
– Vitamin C promotes neurogenesis, neuronal differentiation, and synaptic plasticity, which are important for brain development and function.[2]
– It helps regulate calcium homeostasis and signaling in the brain, which is crucial for neuronal excitability and neuroprotection.[2]
Research indicates that vitamin C levels tend to decline with age and may be lower in certain neurological conditions like Alzheimer’s disease.[1][3][4][5] Supplementation with high-dose vitamin C has been shown to reduce amyloid plaque burden and improve pathological changes in animal models of Alzheimer’s.[5]
Overall, the evidence suggests vitamin C plays a vital role in maintaining healthy brain function, and ensuring adequate vitamin C status may help protect the brain, especially during aging and neurodegeneration.[1][2][3][4][5]
Citations:
[1] https://www.frontiersin.org/articles/10.3389/fnint.2020.00047/full
[2] https://www.mdpi.com/2076-3921/12/2/231
[3] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5622720/
[4] https://www.med.upenn.edu/ngg/assets/user-content/documents/journal-club-2023-2024/reduced-morris-blanco-2022-high-dose-vitamin-c-prevents-second-002.pdf
[5] https://www.nature.com/articles/cddis201426
Niacin for brain protection
Niacin, also known as vitamin B3, has several potential benefits for brain health and protection:
Niacin helps protect the brain from age-related cognitive decline. Studies have found that higher dietary intake of niacin is associated with a reduced risk of Alzheimer’s disease and improved cognitive function with aging.[1][2][3] Niacin is required to form nicotinamide adenine dinucleotide (NAD), a vital molecule for cellular functions, and NAD levels decline with aging. Supplementation with niacin may help slow down cellular aging in the brain.[1]
Niacin protects brain cells from stress and injury. It promotes the growth, development, and survival of brain cells (neurons), especially after injury or oxygen stress.[1][5] Niacin has shown benefits in animal models of traumatic brain injury, stroke, and other brain insults, often when combined with other compounds.[5]
Niacin may help treat certain psychiatric disorders. Some research suggests niacin deficiency may be linked to conditions like schizophrenia, and niacin supplementation may help manage symptoms in these disorders by restoring mitochondrial energy metabolism and neurotransmitter balance.[5][3]
However, more research is still needed to fully understand niacin’s mechanisms and optimal therapeutic applications for brain health and protection. Consulting a healthcare provider is recommended before taking niacin supplements, as high doses can cause side effects.[4]
[1] Niacin slows aging and promotes brain health according to the information provided in[1].
[2] Higher dietary intake of niacin is associated with reduced risk of Alzheimer’s disease, as stated in[1][2][3].
[3] Niacin may help treat certain psychiatric disorders like schizophrenia, as discussed in[5][3].
[4] High doses of niacin supplements can cause side effects, so consulting a healthcare provider is recommended, as mentioned in[4].
[5] Niacin protects brain cells from stress and injury, as described in[1][5].
Citations:
[1] https://www.medicinenet.com/what_does_niacin_do_7_benefits_and_5_side_effects/article.htm
[2] https://medicine.iu.edu/news/2022/03/niacin-alzheimers-research
[3] https://themindfulsteward.com/wellness/the-cognitive-benefits-of-niacin-vitamin-b3/
[4] https://www.healthline.com/nutrition/niacin-benefits
[5] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6412771/
Low omega-6 PUFA intake for brain protection
Low omega-6 PUFA intake may be beneficial for brain protection, according to the search results:
Polyunsaturated fatty acids (PUFAs) are essential for brain development and function. The ratio of omega-3 to omega-6 PUFAs is important, as it influences neurotransmission and other processes vital for normal brain function.[2]
Dietary deficiencies of long-chain PUFAs during brain development can impair neurodevelopment and cause permanent damage.[1] Increasing omega-3 PUFAs like EPA and DHA, while lowering omega-6 PUFAs like linoleic acid (LA), may provide optimal protection against conditions like depression.[1][3]
Some studies have found a negative relationship between omega-6 PUFA (specifically LA) intake and cognitive function.[4][5] This suggests that lowering omega-6 PUFA intake, particularly LA, may be beneficial for brain health and protection.[1][4]
In summary, the evidence indicates that a diet lower in omega-6 PUFAs, especially LA, and higher in omega-3 PUFAs like EPA and DHA, may be optimal for brain development and protection.[1][2][3]
Citations:
[1] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5445635/
[2] https://journals.sagepub.com/doi/pdf/10.1177/070674370304800308
[3] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8065891/
[4] https://www.nature.com/articles/s41538-019-0061-9
[5] https://nutritionj.biomedcentral.com/articles/10.1186/s12937-020-00547-7
High omega-3 PUFA intake for brain protection
Omega-3 polyunsaturated fatty acids (PUFAs), particularly docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), have been shown to have beneficial effects on brain function and structure:
– Omega-3 PUFAs are essential components of neuronal cell membranes, comprising up to 40% of total brain fatty acids. They are crucial for normal brain development and function.[1][2]
– Higher omega-3 PUFA levels, as measured by the omega-3 index in red blood cells, are associated with larger hippocampal volumes and better abstract reasoning abilities in middle-aged adults.[3] The hippocampus is important for learning and memory.
– Omega-3 PUFA supplementation, especially EPA, has been linked to improvements in mood disorders, while DHA is more strongly associated with benefits in neurodegenerative conditions like Alzheimer’s disease.[2]
– In older adults with mild cognitive impairment, supplementation with omega-3s from fish oil has been shown to improve memory and learning performance.[4]
– The omega-3 fatty acids have anti-inflammatory properties and may help protect the brain from the detrimental effects of a high saturated fat diet.[5]
In summary, the evidence suggests that maintaining adequate omega-3 PUFA intake, particularly through dietary sources like fatty fish, may help preserve brain structure and function, especially as we age. Omega-3 supplementation may also provide benefits for those with mild cognitive impairments.[1][2][3][4][5]
Citations:
[1] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9641984/
[2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4404917/
[3] https://news.uthscsa.edu/study-links-omega-3s-to-improved-brain-structure-cognition-at-midlife/
[4] https://www.healthline.com/nutrition/omega-3-fish-oil-for-brain-health
[5] https://www.medicalnewstoday.com/articles/saturated-fat-may-interfere-with-creating-memories-in-the-aged-brain
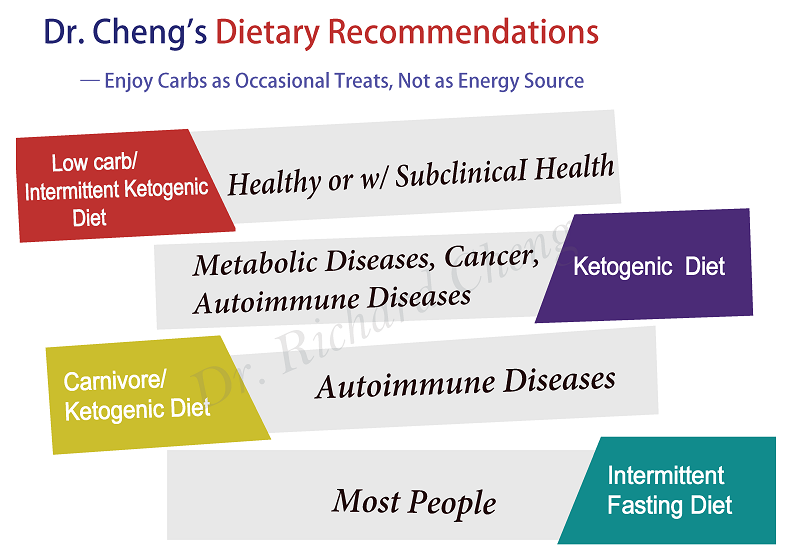
Low carb/ketogenic diet and intermittent fasting for brain protection
The search results provide evidence that a low-carb ketogenic diet and intermittent fasting can have neuroprotective effects and benefits for brain health:
– The ketogenic diet, which is high in fat and low in carbohydrates, has shown promise in animal models and some clinical studies for protecting the brain against damage and improving cognitive function in conditions like Alzheimer’s disease, Parkinson’s disease, epilepsy, and traumatic brain injury.[1][3][4]
– The mechanisms by which the ketogenic diet may confer neuroprotection include increasing resistance to metabolic stress, enhancing alternative energy substrates like ketone bodies, and stimulating mitochondrial biogenesis.[3]
– Intermittent fasting, which involves periods of calorie restriction alternating with normal food intake, has also demonstrated potential benefits for brain health and cognitive function in animal studies. It may work through metabolic, cellular, and circadian mechanisms.[2]
– While the evidence is still limited, some studies have found intermittent fasting may help with conditions like epilepsy, Alzheimer’s, Parkinson’s, and mood/anxiety disorders.[2]
– The ketogenic diet and intermittent fasting appear to have complementary mechanisms of action in supporting brain function, such as elevating ketone bodies as an alternative brain fuel source.[1][2]
In summary, the available research indicates that a low-carb ketogenic diet and intermittent fasting regimens may offer neuroprotective benefits and help maintain or improve brain health, though more longitudinal studies and clinical trials are still needed to fully understand their effects.[1][2][4]
Citations:
[1] https://www.frontiersin.org/articles/10.3389/fnut.2021.782657/full
[2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8470960/
[3] https://www.ncbi.nlm.nih.gov/books/NBK209323/
[4] https://news.stonybrook.edu/featuredpost/low-carb-diet-could-boost-brain-health-study-finds/
[5] https://biology.ucdavis.edu/news/keto-diets-muscle-and-brain-boost
BHRT (Bio-Identical Hormone Replacement Therapy) for brain protection
BHRT, or bioidentical hormone replacement therapy, may offer some protection against brain fog, cognitive decline, and even Alzheimer’s disease in women, but the timing and type of BHRT used is crucial.
The key points are:
– BHRT with estrogen and progesterone can help restore hormonal balance and alleviate brain fog and poor focus during perimenopause and menopause.[1] The pellet form of BHRT is convenient and allows for steady hormone release.
– Estrogen has been shown to have neuroprotective effects, promoting neuronal growth, reducing inflammation, and supporting brain function.[2][3] Estrogen-only BHRT in midlife (40s-50s) may reduce the risk of dementia by up to 32%.[5]
– However, starting BHRT too late, after age 65 or more than 10 years after menopause, may not provide the same benefits and could even increase the risk of dementia, especially if using a combination of estrogen and progesterone.[4][5]
– The timing of BHRT initiation appears crucial, with the “critical window” hypothesis suggesting BHRT is most beneficial when started around the time of menopause.[4]
– BHRT may be particularly helpful for women who are APOE4 carriers, a genetic risk factor for Alzheimer’s, as it has been associated with larger brain volumes in these individuals.[4]
In summary, BHRT can be a promising approach to protect brain health, but the optimal timing and formulation is important. Consulting with a provider experienced in BHRT is recommended to determine if it is the right choice.[1][3]
Citations:
[1] https://evexiasdenver.com/bhrt-pellets-for-brain-fog-and-poor-focus-during-perimenopause/
[2] https://www.lifeextension.com/magazine/2015/2/heal-traumatic-brain-injury-with-bioidentical-hormones
[3] https://www.hybridmedicalsolution.com/in-the-media/can-bhrt-reduce-the-risk-of-alzheimers-in-women/
[4] https://alzres.biomedcentral.com/articles/10.1186/s13195-022-01121-5
[5] https://www.cnn.com/2023/11/02/health/hormone-replacement-dementia-wellness/index.html